Somatotopic organization among parallel sensory pathways that promote a grooming sequence in Drosophila
Figures
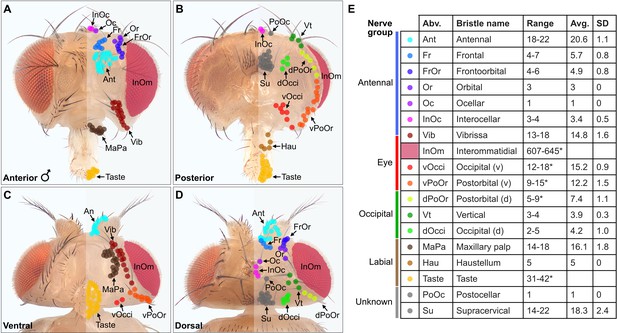
Classification and quantification of D. melanogaster head bristles.
(A–D) Bristles on the anterior (A), posterior (B), ventral (C), and dorsal (D) male head. The bristles on the right half are marked with color-coded dots to indicate their classification. Bristle names are abbreviated (Abv.), and full names and color codes are listed in (E). (E) Quantification of bristle populations on the male head (per half). Range indicates the lowest and highest number of bristles counted across individuals for each population (N=8). Bristle number average (Avg.) and standard deviation (SD) across individuals for each population are shown. Bristle counting was facilitated using color-coded depth maps (examples shown in Figure 1—figure supplement 2). Quantification of bristles on female heads and male/female comparisons are shown in Figure 1—figure supplement 3. See Supplementary file 1 for bristle counts for each head and Supplementary file 2 for image stack download links for each head. *InOm and Taste bristle number ranges are based on published data while dPoOr, PoOr, and Occi bristles were counted using confocal microscopy (see Materials and methods). Bristles are organized into nerve groups based on the nerve each bristle’s corresponding bristle mechanosensory neuron (BMN) projects through to enter the brain (evidence shown in Figure 2). Dorsal (d) and ventral (v).

Hypothesized grooming circuit architecture features somatotopically organized parallel mechanosensory pathways.
(A, B) The architecture elicits aimed grooming of specific locations of the head and body (A) and exerts hierarchical suppression in the grooming sequence (B). (A) Stimulation of a single location (e.g. antennae) by a mechanical stimulus such as dust is detected by local mechanosensory neurons. These neurons connect with distinct postsynaptic circuits that elicit aimed grooming of their respective locations. We referred to these parallel mechanosensory pathways as ‘grooming modules’ in Seeds et al., 2014. The present study focuses on the organization among bristle mechanosensory neurons (BMNs) that elicit aimed grooming of different locations on the head, such as the eyes (red), proboscis (orange), and antennae (aqua). The body locations grooming pathway (gray) represents a continuation of the parallel architecture for any given body location, such as the abdomen or wings. (B) Being coated in dust stimulates multiple locations, inducing competition among the pathways that elicit mutually exclusive grooming movements. These movements are prioritized through hierarchical suppression (unidirectional ball and stick connections between circuits). For illustration simplicity, only the nearest-neighbor connections are shown. In the hypothesized architecture, each circuit suppresses all the subordinate circuits in the hierarchy. For example, eye grooming occurs first in the sequence because it suppresses all later movements, such as grooming of the proboscis, antennae, and body locations. (C) We hypothesize that mechanosensory neurons from different head locations project to distinct somatotopically organized zones in the ventral brain. Mechanosensory neurons on the antennae (Johnston’s organ neurons [JONs]) and eyes (BMNs) are reported to elicit aimed grooming, and BMNs at other locations are hypothesized to also elicit grooming. Mechanosensory neuron projections in the brain are hypothesized to connect with postsynaptic circuits (boxes) that elicit grooming through descending pathways that activate movement pattern generators in the ventral nerve cord.
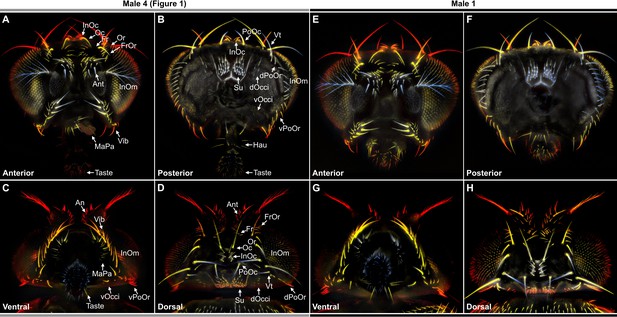
Color-coded depth maps of the head.
(A–H) Example depth maps that were constructed from image Z-stacks (see Materials and methods). Shown are the anterior (A, E), posterior (B, F), ventral (C, G), and dorsal (D, H) views of two different male heads. (A–D) Head shown in Figure 1 (Male 4). (E–H) Example of a different male head (Male 1). Colors indicate closer features in light blue, and increasingly more distant features in white, yellow, and dark red. Bristle names are indicated using abbreviations, whose full names are listed in Figure 1E.
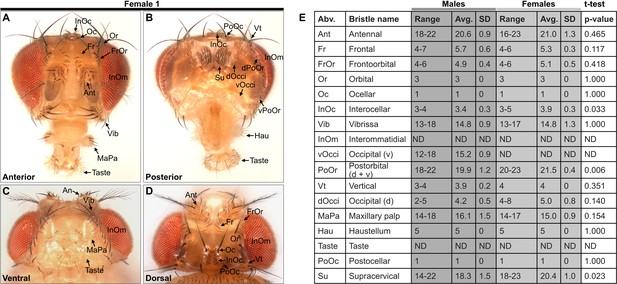
Comparison of bristle numbers on male and female heads.
(A–D) Bristles on an anterior (A), posterior (B), ventral (C), and dorsal (D) female head (Female 1). (E) Table shows quantification of bristles on male and female heads and male/female comparisons (male N=8, female N=4). Bristle numbers for each population are for one half of the head. Range, average (Avg.), and standard deviation (SD) are shown as described in Figure 1. A Student’s t-test (two-tailed) was performed to compare the male/female bristle populations. InOc, PoOr (d+v), and Su bristles show a t-test p≤0.05, however the Bonferroni-adjusted α-value is 0.004 (14 comparisons). See Supplementary file 1 for bristle counts for each head. Note that the PoOr (d+v) bristles were counted as a single group for comparing bristle numbers between males and females. Not determined (ND). See Supplementary file 2 for image stack download links for each head used in this analysis.
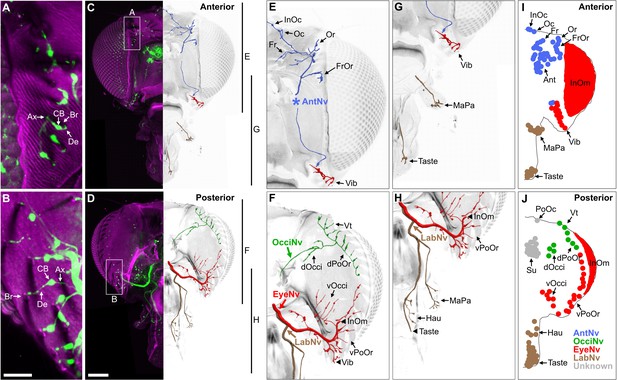
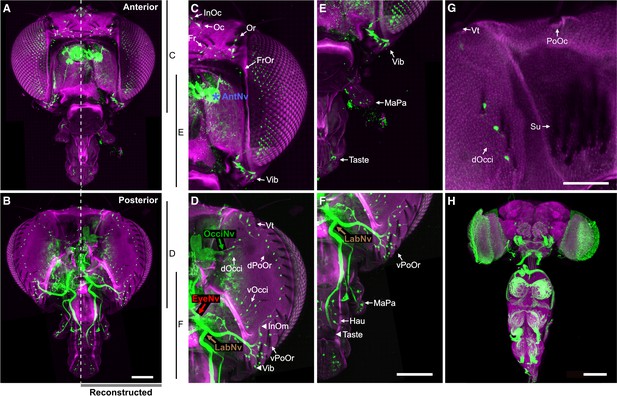
Bristle mechanosensory neurons (BMNs) on the head project through specific nerves.
(A–D) Confocal Z-stack maximum intensity projections of the anterior (A, C) and posterior (B, D) head in which the driver line R52A06-GAL4 drives expression of GFP in BMNs (green). Cuticle is magenta. (A, B) Magnified views of the boxed areas indicated in (C) and (D). The dendrite (De), axon (Ax), cell body (CB), and innervated bristle (Br) of a BMN are indicated in each panel. (C, D) The left half of the head is shown as a maximum projection, while Z-stack-reconstructed BMNs are shown for the right half. Maximum projections of the right half of the head is shown in Figure 2—figure supplement 1A–F. (E–H) Magnified images of the reconstructions. The magnified areas are indicated by vertical lines on the right in (C) and (D). Reconstructed BMNs are color-coded and labeled according to the nerve that they project through: AntNv (blue); OcciNv (green); EyeNv (red); LabNv (brown). Unreconstructed portion of the antennal nerve is indicated by an asterisk. Innervated bristles are indicated with black arrows. Black arrowheads in (F) and (H) indicate partially reconstructed axons of BMNs innervating the InOm, Vib, and Taste bristles. Scale bars: 25 µm (B), 100 µm (D). (I, J) Summary of bristles innervated by BMNs that belong to particular nerve groups on the anterior (I) and posterior (J) head. Nerve groups also listed in Figure 1E, and Supplementary file 2 provides confocal Z-stack download links.
R52A06-GAL4 expression in head bristle mechanosensory neurons (BMNs).
(A, B) Maximum intensity projections of the left and right halves of the anterior (A) and posterior (B) heads that are shown in Figure 2C and D. BMNs are green and the cuticle is magenta. The BMNs on the right side of the midline (dotted line) for either the anterior or posterior heads were reconstructed and shown in Figure 2C and D (right). Anterior and posterior images are from two different heads. (C–F) Magnified images of A and B. Magnified areas are indicated by the vertical lines. The different nerves are labeled with colored arrows. Location of unreconstructed portion of AntNv is indicated by an asterisk. Innervated bristles are indicated with white arrows. White arrowheads in D and F indicate partially reconstructed axons of BMNs innervating the InOm, Vib, and Taste bristles. (G) Posterior region of the head that includes the PoOc, Su, and dOcci bristles. Note that there are no GFP-labeled BMNs innervating the PoOc and Su bristles. (H) Brain and ventral nerve cord of R52A06-GAL4 expressing GFP and immunostained for GFP (green) and Bruchpilot (magenta) to label the neuropile. Scale bars all indicate 100 µm. Supplementary file 2 provides confocal Z-stack download links.
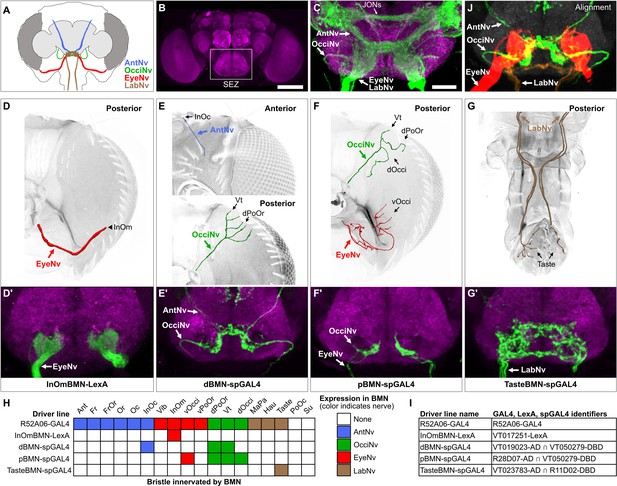
Head bristle mechanosensory neurons (BMNs) project into the ventral brain region called the subesophageal zone (SEZ).
(A) Schematic of BMNs projecting from different nerves into the SEZ. (B) Anterior view of the brain immunostained for Bruchpilot (magenta) to visualize the neuropile. White box indicates the SEZ. Scale bar, 100 µm. (C) Image of the SEZ in which R52A06-GAL4 expressed GFP in BMNs and Johnston’s organ neurons (JONs). Brains were immunostained for GFP (green) and Bruchpilot (magenta). BMN nerves and JONs are labeled. Scale bar, 25 µm. (D–G) Driver lines that label BMNs from different nerves. Reconstructed BMNs on half of the head that are labeled by the following driver lines: InOmBMN-LexA (D), dBMN-spGAL4 (E), pBMN-spGAL4 (F), and TasteBMN-spGAL4 (whole proboscis shown) (G). Images of the heads used for each reconstruction are shown in Figure 3—figure supplement 1A–D. Reconstructed neurons are color-coded and labeled as described in Figure 2. (D’–G’) SEZ projections of BMNs from both halves of the head that are labeled by InOmBMN-LexA (D’), dBMN-spGAL4 (E’), pBMN-spGAL4 (F’), and TasteBMN-spGAL4 (G’). (H) Table of BMNs innervating specific bristles that are labeled by each driver line, indicated by box shading (numbers of labeled BMNs innervating different bristles shown in Figure 3—figure supplement 1E). Shaded color indicates the nerve that each BMN projects through. (I) Driver line names and identifiers. (J) Shown in the upper right corner of the figure are the aligned expression patterns of InOmBMN-LexA (red), dBMN-spGAL4 (green), and TasteBMN-spGAL4 (brown). Supplementary file 2 provides confocal Z-stack download links.
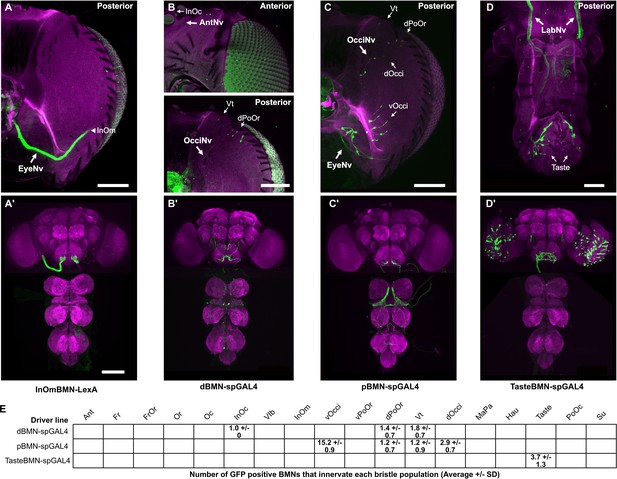
Driver line expression in head bristle mechanosensory neuron (BMNs).
(A–D’) Maximum intensity projections of heads (A–D) and central nervous systems (CNSs) (A’–D’) expressing GFP in BMNs that innervate different bristles. (A–D) were produced from the same confocal Z-stacks that were used for the BMN reconstructions shown in Figure 3D–G. Magnified views of the subesophageal zones (SEZs) in A’–D’ are shown in Figure 3D’–G’. The expression patterns of the following driver lines are shown: InOmBMN-LexA (A, A’), dBMN-spGAL4 (B, B’), pBMN-spGAL4 (C, C’), and TasteBMN-spGAL4 (D, D’). Scale bars, 100 µm (A–C), 50 µm (D), 100 µm (A’–D’). (E) Table showing the average number of GFP positive BMNs (and SD) that innervate each bristle population from the indicated spGAL4 lines. Supplementary file 2 provides confocal Z-stack download links.
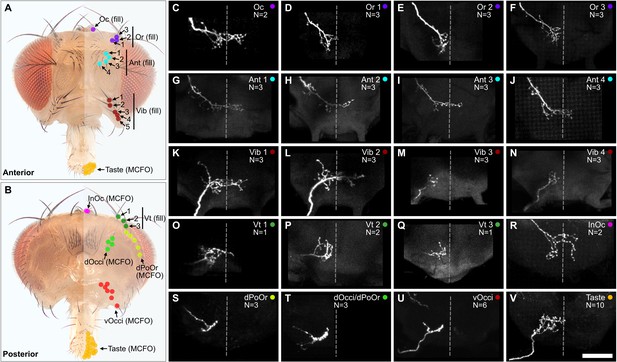
Projections of bristle mechanosensory neurons (BMNs) that innervate specific head bristles.
(A, B) Bristles on the anterior (A) and posterior (B) head whose associated BMNs were labeled using dye fill (C–Q, fill) or multicolor flipout (R–V, MCFO) techniques. (C–V) Subesophageal zone (SEZ) projections of individual BMNs that innervate the bristle indicated in the upper right corner (anterior view). BMNs are oriented as if they are projecting from the right side of the head. Dotted line indicates approximate SEZ midline. Scale bar, 50 µm. (C–Q) BMNs labeled by dye filling. Schematic of the filling technique and whole brain examples shown in Figure 4—figure supplement 1. Filled BMNs innervate the Oc (C), Or (D–F), Ant (G–J), Vib (K–N), and Vt (O–Q) bristles. All fill trials for the different bristles are shown in Figure 4—figure supplement 2, Figure 4—figure supplement 3, Figure 4—figure supplement 4, and Figure 4—figure supplement 5. (R–V) MCFO-labeled BMNs innervate the InOc (R), dPoOr (S), dOcci/dPoOr (T), vOcci (U), and Taste (V) bristles. BMNs were MCFO labeled using the following driver lines: dBMN-spGAL4 (R, S), pBMN-spGAL4 (T, U), and TasteBMN-spGAL4 (V). All MCFO trials for the different bristles are shown in Figure 4—figure supplement 6, Figure 4—figure supplement 7, and Figure 4—figure supplement 8. The number (N) of fill or MCFO trials obtained for each BMN is indicated in the upper right corner. Supplementary file 2 provides confocal Z-stack download links.
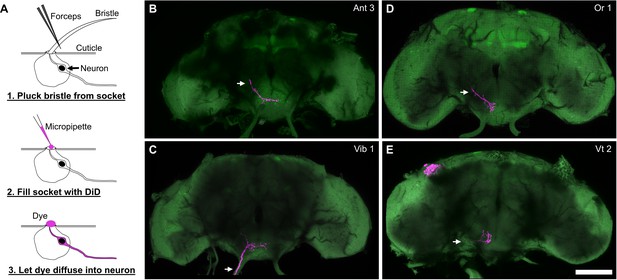
Overview of the dye filling technique and whole brain examples.
(A) Schematic of the dye filling technique used for labeling bristle mechanosensory neurons (BMNs) from specific bristles. BMNs were labeled with the anterograde dye, DiD. Each bristle was plucked from a head and DiD was pipetted onto the exposed socket to label the BMN innervating that bristle. (B–E) Whole brain examples of four fills shown in Figure 4. Examples are from Ant 3 (B), Vib 1 (C), Or 1 (D), and Vt 2 (E) bristle socket fills. Each filled BMN is magenta and the brain neuropile is labeled with pan neuronally expressed nSyb.GFP in green. Scale bar, 100 µm. Supplementary file 2 provides confocal Z-stack download links.

Different fill trials for Oc and Or bristles.
(A) Oc and Or bristles whose associated bristle mechanosensory neurons (BMNs) were labeled by dye filling are indicated with labeled dots (dorsal view). The boxed area in the top image is shown magnified in the bottom image. (B–L) Anterior view of the subesophageal zone (SEZ) projections of individual BMNs that innervate the Oc (B, C), Or 1 (D–F), Or 2 (G–I), and Or 3 (J–L) bristles. Two or three different flies were tested for each bristle (fly number indicated in upper right corner). Asterisk indicates the BMN example that is shown in Figure 4. BMNs are oriented as if they are projecting from the right side of the head. Scale bar, 50 µm. Dotted line indicates approximate SEZ midline. Supplementary file 2 provides confocal Z-stack download links.

Different fill trials for Ant bristles.
(A) Ant bristles whose associated bristle mechanosensory neurons (BMNs) were labeled by dye filling are indicated with labeled dots (anterior view). The boxed area in the top image is shown magnified in the bottom image. (B–M) Anterior view of the subesophageal zone (SEZ) projections of individual BMNs that innervate the Ant 1 (B–D), Ant 2 (E–G), Ant 3 (H–J), and Ant 4 (K–M) bristles. Three different flies were tested for each bristle (fly number indicated in upper right corner). Asterisk indicates the BMN example that is shown in Figure 4. Scale bar, 50 µm. Dotted line indicates approximate SEZ midline. Supplementary file 2 provides confocal Z-stack download links.
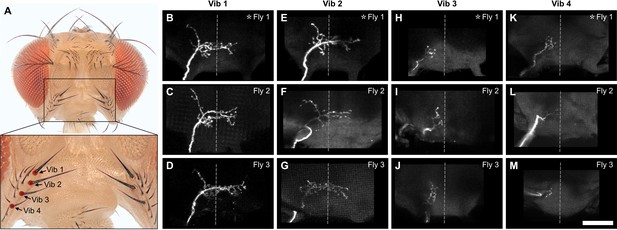
Different fill trials for Vib bristles.
(A) Vib bristles whose associated bristle mechanosensory neurons (BMNs) were labeled by dye filling are indicated with labeled dots (anterior view). The boxed area in the top image is shown magnified in the bottom image. (B–M) Anterior view of the subesophageal zone (SEZ) projections of individual BMNs that innervate the Vib 1 (B–D), Vib 2 (E–G), Vib 3 (H–J), and Vib 4 (K–M) bristles. Three different flies were tested for each bristle (fly number indicated in upper right corner). Asterisk indicates the BMN example that is shown in Figure 4. Scale bar, 50 µm. Dotted line indicates approximate SEZ midline. Supplementary file 2 provides confocal Z-stack download links.
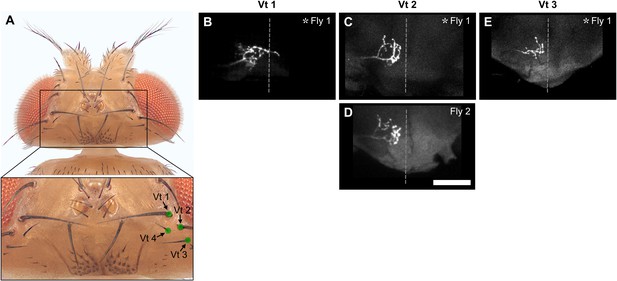
Different fill trials for Vt bristles.
(A) Vt bristles whose associated bristle mechanosensory neurons (BMNs) were labeled by dye filling are indicated with labeled dots (dorsal view). The boxed area in the top image is shown magnified in the bottom image. (B–E) Anterior view of the subesophageal zone (SEZ) projections of individual BMNs that innervate the Vt 1 (B), Vt 2 (C, D), and Vt 3 (E) bristles. One or two different flies were tested for each bristle (fly number indicated in upper right corner). Asterisk indicates the BMN example that is shown in Figure 4. Scale bar, 50 µm. Dotted line indicates approximate SEZ midline. Note: Vt 4 was not filled. Supplementary file 2 provides confocal Z-stack download links.
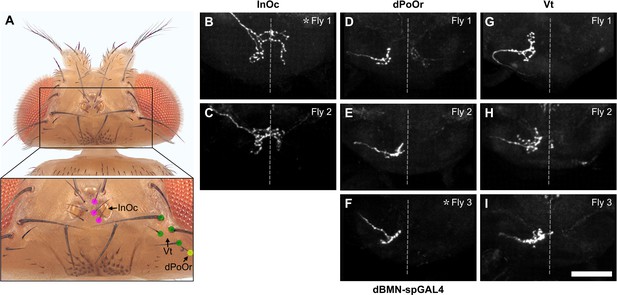
Multicolor flipout (MCFO) trials for bristle mechanosensory neurons (BMNs) innervating the InOc, dPoOr, and Vt bristles.
(A) Bristles whose associated BMNs were labeled by MCFO are indicated with labeled dots (dorsal view). The boxed area in the top image is shown magnified in the bottom image. (B–I) Anterior view of the subesophageal zone (SEZ) projections of individual BMNs labeled by MCFO using dBMN-spGAL4. BMNs shown innervate the InOc (B, C), dPoOr (D–F), and Vt (G–I) bristles. At least two different flies were tested for each BMN (fly number indicated in upper right corner). Asterisk indicates the BMN example that is shown in Figure 4. Scale bar, 50 µm. Dotted line indicates approximate SEZ midline. Supplementary file 2 provides confocal Z-stack download links.
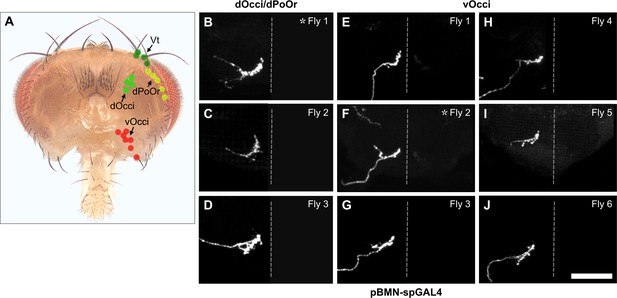
Multicolor flipout (MCFO) labeled trials for bristle mechanosensory neurons (BMNs) innervating the dOcci/dPoOr and vOcci bristles.
(A) Bristles whose associated BMNs were labeled by MCFO are indicated with labeled dots (posterior view). (B–J) Anterior view of the subesophageal zone (SEZ) projections of individual BMNs labeled by MCFO using pBMN-spGAL4. BMNs shown innervate the dPoOr/dOcci (B–D) and vOcci (E–J) bristles. Three or six different flies were tested for each BMN (fly number indicated in upper right corner). Asterisk indicates the BMN example that is shown in Figure 4. Scale bar, 50 µm. Dotted line indicates approximate SEZ midline. Supplementary file 2 provides confocal Z-stack download links.
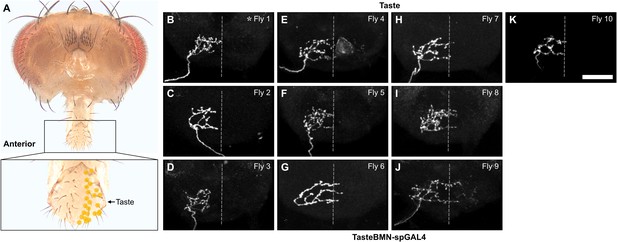
Multicolor flipout (MCFO)-labeled trials for Taste bristles.
(A) Bristles whose associated bristle mechanosensory neurons (BMNs) were labeled by MCFO are indicated with labeled dots (posterior view). The boxed area in the top image is shown magnified in the bottom image. (B–K) Anterior view of the subesophageal zone (SEZ) projections of individual BMNs labeled by MCFO using TasteBMN-spGAL4. BMNs shown innervate the Taste bristles. Ten different flies were tested (fly number indicated in upper right corner). Asterisk indicates the BMN example that is shown in Figure 4. Scale bar, 50 µm. Dotted line indicates approximate SEZ midline. Supplementary file 2 provides confocal Z-stack download links.
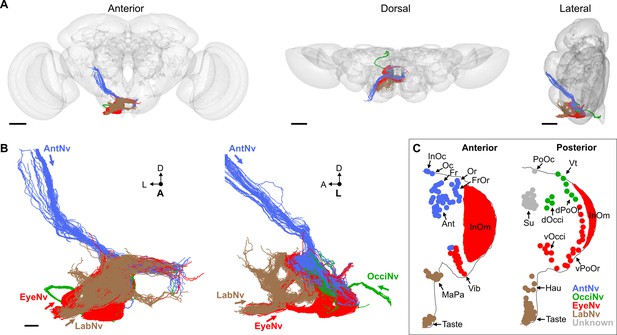
Electron microscopy (EM)-based reconstruction of head bristle mechanosensory neurons (BMNs).
(A) All reconstructed BMNs projecting into the brain from the left side of the head (anterior, dorsal, and lateral views shown). BMN colors correspond to the nerves that they project through, including the AntNv (blue), EyeNv (red), OcciNv (green), and LabNv (brown). Scale bars, 50 µm. (B) Zoomed anterior (left) and lateral (right) views of the BMNs in the subesophageal zone (SEZ). Labeled arrows for each incoming nerve indicate BMN projection direction. Scale bar, 10 µm. (C) Bristles on the anterior (left) and posterior (right) head that are innervated by BMNs in the nerve groups indicated by their color. Figure 5—figure supplement 1 summarizes the EM reconstruction strategy. Sensory neurons that could not be assigned an identity are shown in Figure 5—figure supplement 2.
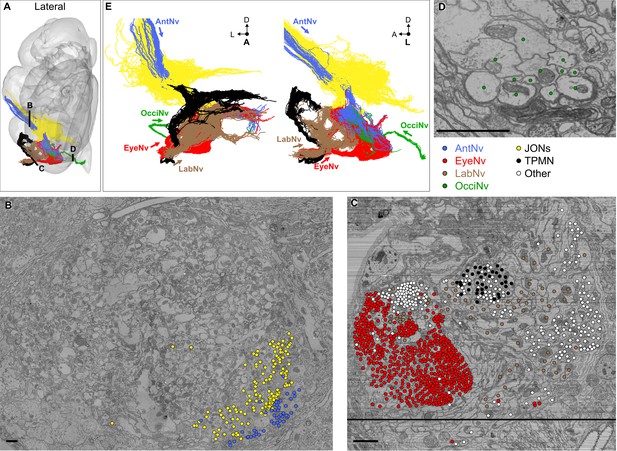
Reconstruction of mechanosensory neurons in different head nerves.
(A) Locations in each nerve where different segmented neurons in the electron microscopy (EM) volume were seeded for proofreading and editing (black lines). (B–D) EM sections through each nerve at the locations shown in A with seeded neurons indicated with dots. Shown are AntNv (B), EyeNv/LabNv (C), and OcciNv (D) sections. Dot colors indicate the seeded neuron type, including BMNs projecting through the AntNv (blue), EyeNv (red), LabNv (brown), OcciNv (green), Johnston’s organ neurons (JONs) (yellow), taste peg mechanosensory neurons (TPMNs) (black), or other neurons (white). All neuron segments were seeded for each nerve, with the following exceptions: (1) The Eye/LabNv had a bundle of soma tracts from an interneuron hemilineage crossing the seed plane that was excluded based on the morphology of their initial segmentation, and (2) previously reconstructed JONs were excluded when seeding the AntNv (B, upper left), leaving a ventral-medial area of the nerve with previously undocumented neurons (bottom right). Scale bars, 2 µm. (E) Anterior (left) and lateral (right) views of all reconstructed head mechanosensory neurons, including BMNs, JONs, and TPMNs (colors same as A–D). Arrows for each incoming nerve indicate projection direction.

Reconstructed sensory neurons that could not be assigned an identity (unknown sensory neurons).
(A–Y) Subesophageal zone (SEZ) projections (dorsal views) of unknown sensory neurons 001 (A), 002 (B), 003 (C), 004 (D), 005 (E), 006 (F), 007 (G), 008 (H), 009 (I), 010 (J), 011 (K), 012 (L), 013 (M), 014 (N), 015 (O), 016 (P), 017 (Q), 018 (R), 019 (S), 020 (T), 021 (U), 022 (V), 023 (W), 024 (X), and 025 (Y). Unknown sensory neurons project through the AntNv (A–H) or the EyeNv/LabNv (I–Y). Unknown neurons in S–U send their axons into the neck connective, and possibly descend to the ventral nerve cord.
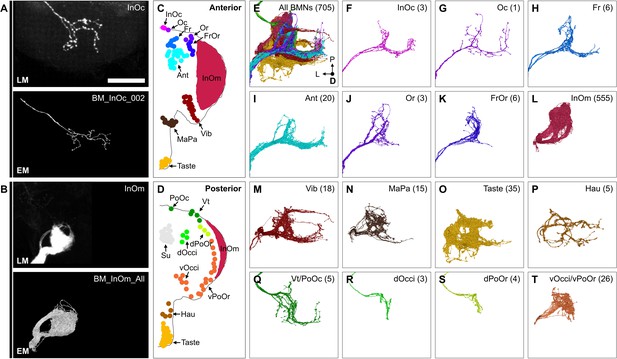
Bristle mechanosensory neuron (BMN) types that innervate specific head bristles.
(A–B) Examples of matching light microscopy (LM) imaged BMN projections with their corresponding electron microscopy (EM)-reconstructed BMNs, including BM-InOc neurons (A) and BM-InOm neurons (B). Top panels show representative LM images of labeled BMNs that innervate the bristle indicated in the top right corner (anterior subesophageal zone [SEZ] views as shown in Figure 4). The individual BM-InOc neuron was labeled by dye filling using DiD while the collective projections of the BM-InOm neurons were labeled using the driver line InOmBMN-LexA expressing GFP. Bottom panels show the EM-reconstructed BMN types indicated in the top right corner. Shown is a representative example of a BM-InOc neuron (A) and all reconstructed BM-InOm neurons (B). Scale bar, 50 µm. Examples for all LM and EM matched BMNs are shown in Figure 6—figure supplement 2. Additional evidence used for assigning the different BMN types is shown in Figure 6—figure supplement 1, Figure 6—figure supplement 3, and Figure 6—figure supplement 4. (C–D) Different bristle populations indicated by labeled and colored dots are innervated by BMNs shown in E–T. The anterior (C) and posterior (D) head are shown. (E–T) Reconstructed SEZ projections of BMN types that are labeled and plotted in colors indicating the bristles that they innervate. Shown are the dorsal views of all BMNs (E), BM-InOc (F), BM-Oc (G), BM-Fr (H), BM-Ant (I), BM-Or (J), BM-FrOr (K), BM-InOm (L), BM-Vib (M), BM-MaPa (N), BM-Taste (O), BM-Hau (P), BM-Vt/PoOc (Q), BM-dOcci (R), BM-dPoOr (S), and BM-Occi/vPoOr (T) neurons. The number of reconstructed BMNs for each type is indicated.
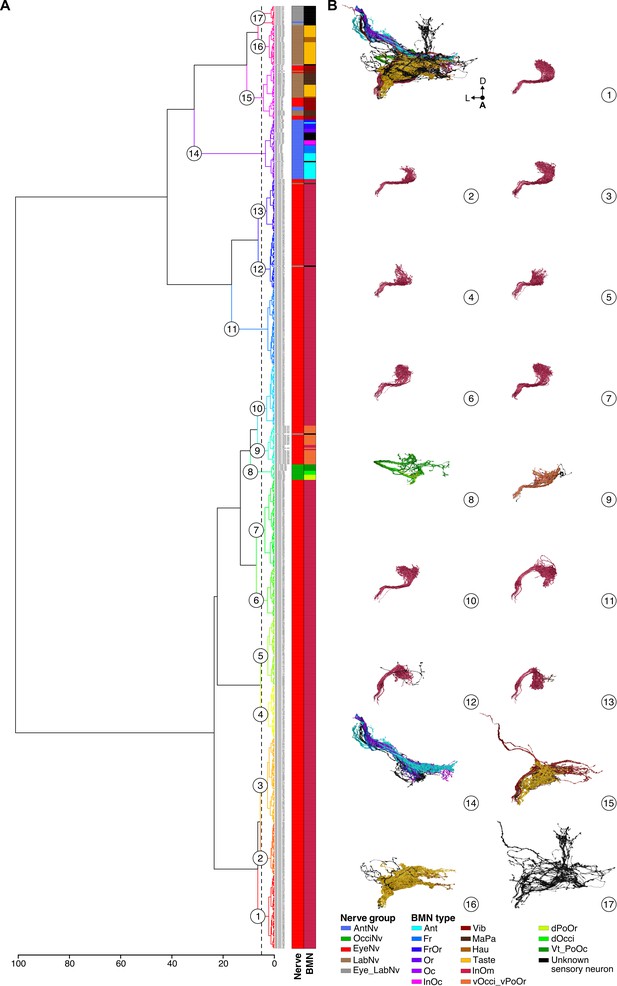
NBLAST clustering of bristle mechanosensory neurons (BMNs).
(A) Dendrogram of Ward clustered NBLAST similarity scores of all reconstructed sensory neurons. Identified nerve projection groups and BMN type assignments are color-coded in the bars to the right (color IDs shown in the bottom right corner). The dendrogram was cut at H=5, resulting in 17 clusters indicated by numbers on the dendrogram. Supplementary file 3 shows the NBLAST clusters when the dendrogram was cut at H=1, at which most of the BMN types are clustered individually. (B) Morphology of the BMNs in each NBLAST cluster shown in A, indicated by the same number (color-coded by type).
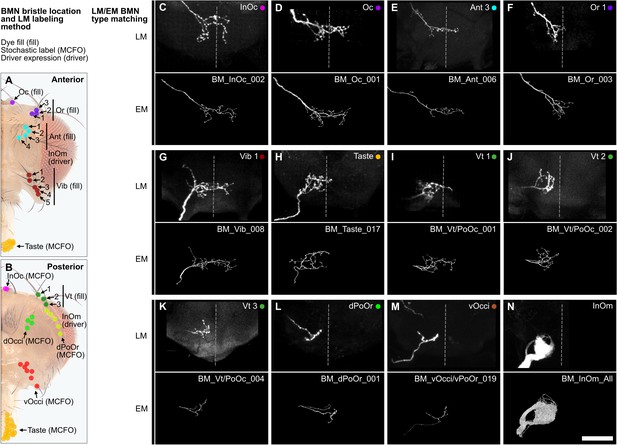
Matching electron microscopy (EM)-reconstructed bristle mechanosensory neuron (BMN) projections with light microscopy (LM) imaged BMNs that innervate specific bristles.
(A–B) Bristles on the anterior (A) and posterior (B) head whose associated BMNs were labeled by dye filling (fill), stochastic labeling (multicolor flipout [MCFO]), or full pattern driver line expression (driver). (C–N) Top panels show representative images of the subesophageal zone (SEZ) projections of labeled and LM imaged BMNs that innervate the bristle indicated in the top right corner (anterior view). All fill and MCFO trials for the different bristles are shown in Figure 4—figure supplements 2–8. Bottom panels show representative examples of the EM-reconstructed BMN types (types indicated in the top right corner). BMN types and their LM labeling methods are BM-InOc (MCFO) (C), BM-Oc (fill) (D), BM-Ant (fill) (E), BM-Or (fill) (F), BM-Vib (fill) (G), BM-Taste (MCFO) (H), BM-Vt/PoOc (fill) (I), BM-Vt/PoOc (fill) (J), BM-Vt/PoOc (fill) (K), BM-dPoOr (MCFO) (L), BM-Occi/PoOr (MCFO) (M), and BM-InOm (driver) (N). Note that the BM-InOm neurons shown in N (top panel) are those labeled in the full expression pattern of the driver line InOmBMN-LexA. The bottom panel of N includes all EM-reconstructed BM-InOm neurons. Scale bar, 50 µm.
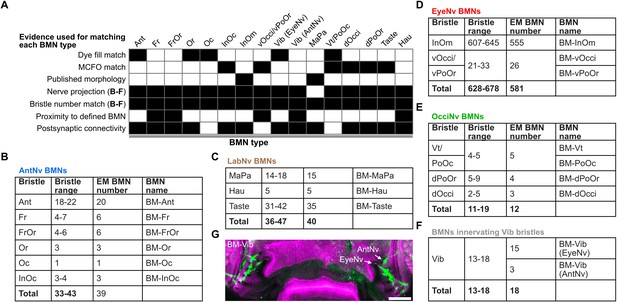
Evidence used to match the electron microscopy (EM)-reconstructed bristle mechanosensory neurons (BMNs) with their bristles.
(A) NBLAST clusters were assigned as types that innervate particular bristle populations based on: (1) comparison of their morphology with images of dye-filled, multicolor flipout (MCFO)-labeled, or published BMNs, (2) nerve projections, (3) proximity to BMNs with known morphology, and (4) common connectivity with postsynaptic partners. Postsynaptic connectivity clustering is shown in Figure 8—figure supplement 2. The BMN types were validated because their numbers matched the numbers of their corresponding bristles. The mismatch between the reconstructed BM-InOm neurons and the number of InOm bristles is likely due to the latter being an estimate (shown in D). Black shaded boxes indicate which evidence was used to match each BMN type. Detailed descriptions of the evidence that was used for assigning each BMN type can be found in Materials and methods. (B–F) Head bristle counts for each population and the number of reconstructed BMNs for each type. Shown are BMNs projecting through the AntNv (B), LabNv (C), EyeNv (D), and OcciNv (E), and BMNs innervating the Vib bristles (F). (G) R52A06-GAL4 expression in BM-Vib neurons on the head (green). Maximum intensity projections of the ventral head. Cuticle is magenta. BM-Vib neurons (green) that project to the brain through the AntNv or EyeNv are labeled with arrows. Scale bar, 50 µm.
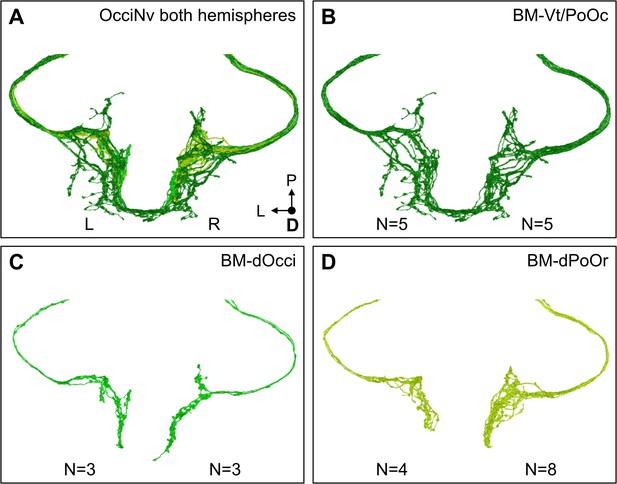
Electron microscopy (EM) reconstruction of OcciNv bristle mechanosensory neurons (BMNs) from both brain hemispheres.
(A) All BMNs entering the brain through the right (R) and left (L) OcciNv, color-coded by the BMN type. (B, C) BMN numbers are consistent across hemispheres for the BM-Vt/PoOc (B) and BM-dOcci (C) neurons (indicated below each hemisphere). (D) Reconstruction of the full occipital nerve on both hemispheres revealed twice as many BM-dPoOr neurons on the right than on the left.
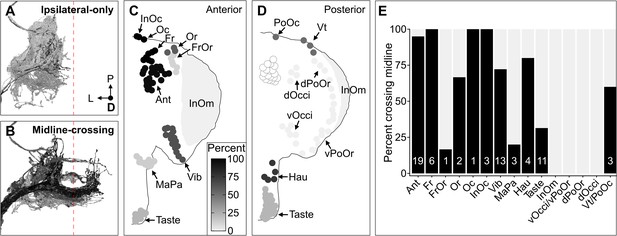
Some head bristle mechanosensory neurons (BMNs) have projections that cross the midline to the contralateral brain hemisphere.
(A–B) BMNs that remain in the ipsilateral brain hemisphere (A) versus those with midline-crossing projections (B), shaded by percent midline-crossing for each type (scale in C). Red dashed line indicates the brain midline. (C, D) Shaded dots on the anterior (C) and posterior (D) head indicate the percent of BMNs innervating each bristle population that are midline-crossing. (E) Bar plots of midline-crossing percentages (numbers of midline-crossing BMNs indicated).
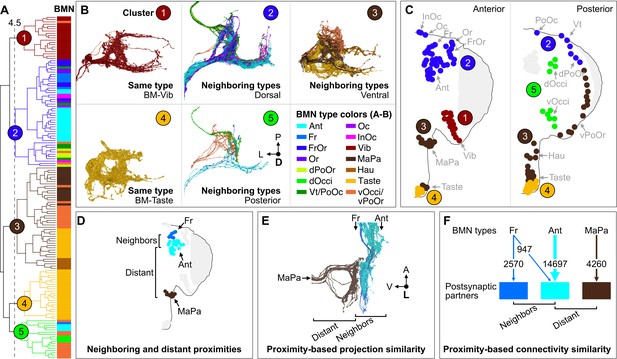
Somatotopy-based postsynaptic connectivity similarity among bristle mechanosensory neuron (BMN) types.
(A) Dendrogram of cosine similarity clustering of BMNs by postsynaptic connectivity similarity. Analysis excludes postsynaptic partners with fewer than six synapses, and the BMN/BMN connections shown in Figure 8—figure supplement 1. Individual BMNs are shown as bars and their types correspond to the colors indicated in B (bottom right). The five clusters are from cut height 4.5 on the dendrogram (dotted line) derived from the comparisons shown in Figure 8—figure supplement 2. (B) Morphologies of BMNs in the indicated clusters (upper right) whose types correspond to the colors shown in the bottom right. (C) Spatial relationships among the clustered BMNs are shown by coloring their bristles (dots) by cluster number on the anterior and posterior head. BMN types in more than one cluster are colored accordingly if at least 20% of that type was in a given cluster (e.g. BM-Taste neurons are in Clusters 3 [37%, brown] and 4 [63%, orange]). Note: the positioning of the colored dots indicating different clusters for Taste and Occi/PoOr bristles is hypothesized based on their proximity to other BMNs in the same cluster. The clusters exemplify different levels of connectivity similarity shown by the dendrogram (A). BMNs showing the highest connectivity similarity innervate the same bristle populations, as exemplified by BM-Vib (Cluster 1) and BM-Taste (Cluster 4) neurons. BMNs that innervate neighboring bristle populations also show high connectivity similarity, including BMNs on the dorsal (Cluster 2), ventral (Cluster 3), and posterior head (Cluster 5). Note: Cluster 5 consists mostly of posterior head BMNs, but also BM-Ant and -Fr neurons on the anterior head, although these BMNs show relatively low cosine similarity with the posterior head BMNs. BM-InOm neurons were analyzed separately (Figure 8—figure supplement 3). (D–F) Summary of BMN somatotopic features. (D) Different BMN types innervate bristles at neighboring and distant proximities. (E, F) BMNs that innervate neighboring bristles project into overlapping zones (E, example of electron microscopy (EM)-reconstructed BM-Fr and -Ant neuron subesophageal zone (SEZ) projections with non-overlapping -MaPa neuron projections) and can show postsynaptic connectivity similarity (F, edge widths based on number of total synapses from a given BMN type to its major postsynaptic partners, edges under 5% of BMN output omitted). Labeled arrows for each BMN type shown in E indicate projection direction.

Bristle mechanosensory neuron (BMN)-type synaptic counts and BMN/BMN connectivity.
(A) Number pre- and postsynaptic connections for each BMN type. Black lines indicate mean synapse number. (B) Synaptic connectivity among BMN types. The average synaptic count for each edge is shown on arrows indicating directionality of the connection (arrow width corresponds to the count). Total count of synapses between two given types was divided by the number of possible edges between the two types.
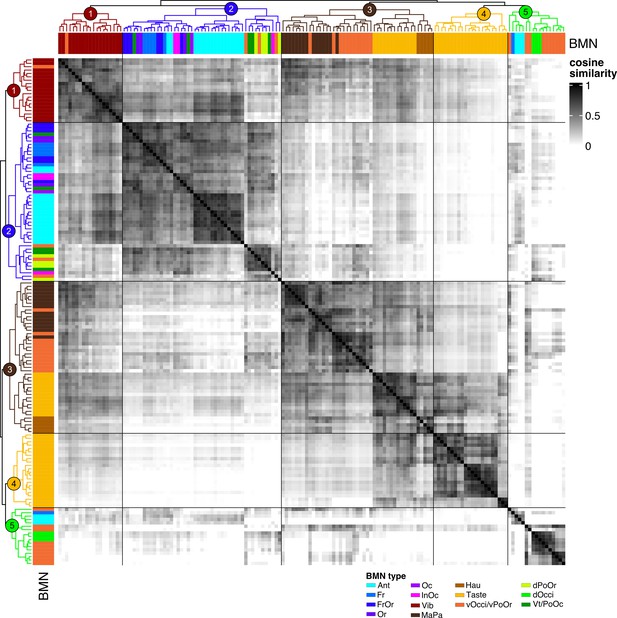
Cosine similarity clustering of bristle mechanosensory neuron (BMN) to non-BMN postsynaptic connectivity.
Heatmap of the cosine similarity among BMNs with the row and column order determined by clustering of the similarity scores. Postsynaptic partners excluded connections to other BMNs and connections with fewer than six synapses. Clustering of the rows and columns is the same as the dendrograms next to bars that indicate BMN type (color-code on bottom right). Dendrogram cut height 4.5 resulted in five clusters (indicated by numbers on the top dendrogram). Morphologies of the neurons in each cluster are shown in Figure 8B.
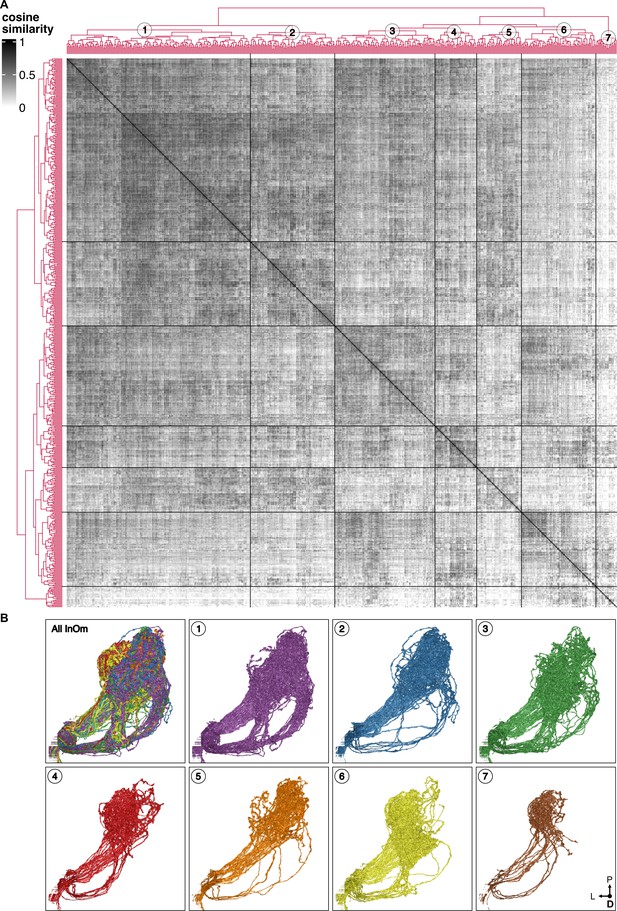
Cosine similarity clustering of BM-InOm neurons in their connectivity with non-bristle mechanosensory neuron (BMN) postsynaptic partners.
(A) Heatmap of BM-InOm neuron cosine similarity with the row and column order determined by clustering of the similarity scores. Postsynaptic partners excluded connections to other BMNs and connections with fewer than two synapses. Clustering of the rows and columns is the same with dendrograms shown on the left and top. Dendrogram cut height 5.6 resulted in seven clusters (indicated by numbers on the top dendrogram). (B) Dorsal view of the neuron morphologies in each cluster (cluster number indicated at the top left), revealed tiling of the BM-InOm innervation area in the brain. Clusters 1–2 mainly innervate an area located medial-dorsally, while Clusters 6–7 innervate a lateral-ventral area. Clusters 3–5 send branches to both areas.
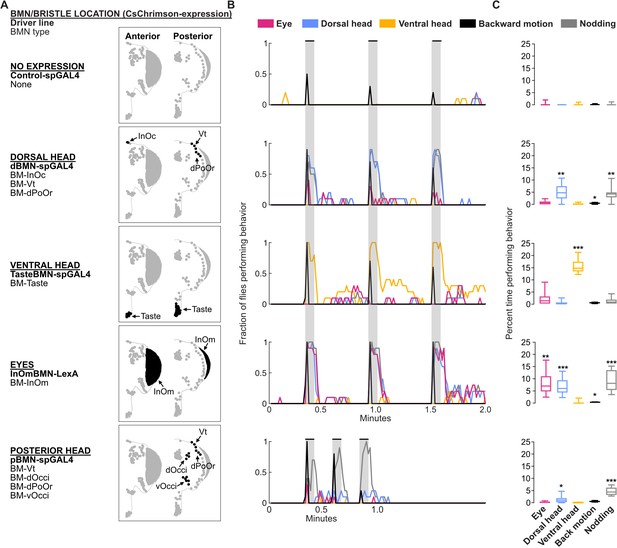
Optogenetic activation of bristle mechanosensory neurons (BMNs) at specific head locations elicits aimed grooming.
(A) Bristles shaded black on the anterior (left) and posterior (right) head are innervated by BMNs that express CsChrimson under control of the indicated driver lines. Control-spGAL4 shows no expression. (B) Histograms of manually annotated video for each line show movements elicited with red-light-induced optogenetic activation. The fraction of flies performing each movement are plotted in 1 s bins (N=10 flies per line). Grooming movements are indicated by different colors, including eye (magenta), dorsal head (blue), and ventral head (orange) grooming. Other elicited movements include backward motion (black) and head nodding (gray). Gray bars indicate a 5 s red-light stimulus. Most driver lines were tested using 30 s interstimulus intervals, while pBMN-spGAL4 elicited more reliable behavior using 10 s intervals. Movements are mutually exclusive except head nodding. Representative experimental trials shown in Video 1, Video 2, Video 3, Video 4, and Video 5. Figure 9—figure supplement 1 shows additional controls and ethograms for individual flies tested. (C) Box plots show the percent time that flies spent performing each movement during the experiment shown in B. Bottom and top of the boxes indicate the first and third quartiles, respectively; median is shown in each box; whiskers show the minimum and maximum values. Asterisks indicate *p, 0.05, **p, 0.001, ***p, 0.0001 from Mann-Whitney U pairwise tests between each experimental line and its corresponding control after application of Bonferroni correction. Figure 9—source data 1 contains numerical data used for producing each box plot.
-
Figure 9—source data 1
Numerical data used for producing each box plot.
Rows correspond to individual flies. Columns indicate the percent time each fly spent performing different movements, including eye, dorsal, and ventral head grooming, nodding, and backward motions.
- https://cdn.elifesciences.org/articles/87602/elife-87602-fig9-data1-v1.xlsx

Ethograms of movements performed with activation of different bristle mechanosensory neurons (BMNs).
Ethograms of manually scored videos showing the movements elicited with red-light-induced activation. Ethograms of individual flies are stacked. Grooming movements (top plots) are indicated by different colors, including eye (magenta), dorsal head (blue), and ventral head (orange) grooming. Other elicited movements (bottom plots) include backward motion (black) and head nodding (gray). Gray bars indicate a 5 s red-light stimulus. Most driver lines were tested using 30 s interstimulus intervals, while pBMN-spGAL4 elicited more reliable behavior using 10 s intervals. Movements are mutually exclusive except head nodding. Gray bars indicate a 5 s red-light stimulus.
Videos
Optogenetic activation of dorsal head bristle mechanosensory neurons (BMNs) elicits aimed dorsal head grooming.
CsChrimson was expressed in BMNs targeted by the dBMN-spGAL4 driver line. Infrared light in the bottom right corner indicates when the red light was on to activate the targeted BMNs. Note that head nodding movements and backward motions are also elicited.
Optogenetic activation of BM-Taste neurons elicits aimed proboscis and ventral head grooming.
CsChrimson was expressed in bristle mechanosensory neurons (BMNs) targeted by the TasteBMN-spGAL4 driver line. Infrared light in the bottom right corner indicates when the red light was on to activate the targeted BMNs.
Optogenetic activation of BM-InOm neurons elicits eye and dorsal head grooming.
CsChrimson was expressed in bristle mechanosensory neurons (BMNs) targeted by the InOmBMN-LexA driver line. Infrared light in the bottom right corner indicates when the red light was on to activate the targeted BMNs. Note that head nodding movements and backward motions are also elicited.
Optogenetic activation of posterior head bristle mechanosensory neurons (BMNs) elicits head nodding.
CsChrimson was expressed in BMNs targeted by the pBMN-spGAL4 driver line. Infrared light in the bottom right corner indicates when the red light was on to activate the targeted BMNs. Note that dorsal head grooming movements are also elicited (not shown in video).
Optogenetic stimulus in control flies.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (D. melanogaster) | R52A06-GAL4 | Jenett et al., 2012 | RRID:BDSC_38810 | |
| Genetic reagent (D. melanogaster) | VT017251-LexA | Hampel et al., 2017 | aka InOmBMN-LexA | |
| Genetic reagent (D. melanogaster) | VT019023-AD | Tirian and Dickson, 2017 | RRID:BDSC_71430 | |
| Genetic reagent (D. melanogaster) | VT050279-DBD | Tirian and Dickson, 2017 | RRID:BDSC_72433 | |
| Genetic reagent (D. melanogaster) | R28D07-AD | Dionne et al., 2017 | RRID:BDSC_70168 | |
| Genetic reagent (D. melanogaster) | VT023783-AD | Tirian and Dickson, 2017 | RRID:BDSC_73261 | |
| Genetic reagent (D. melanogaster) | R11D02-DBD | Dionne et al., 2017 | RRID:BDSC_68554 | |
| Genetic reagent (D. melanogaster) | dBMN-spGAL4 | This paper | Stock contains VT019023-AD and VT050279-DBD | |
| Genetic reagent (D. melanogaster) | pBMN-spGAL4 | This paper | Stock contains R28D07-AD and VT050279-DBD | |
| Genetic reagent (D. melanogaster) | TasteBMN-spGAL4 | This paper | Stock contains VT023783-AD and R11D02-DBD | |
| Genetic reagent (D. melanogaster) | 20XUAS-IVS-mCD8::GFP | Pfeiffer et al., 2010 | RRID:BDSC_32194 | |
| Genetic reagent (D. melanogaster) | 13XLexAop2-IVS-myr::GFP | Pfeiffer et al., 2010 | RRID:BDSC_32209 | |
| Genetic reagent (D. melanogaster) | C155-GAL4, UAS-nSyb.eGFP | Kendal Broadie | RRID:BDSC_6920 | |
| Genetic reagent (D. melanogaster) | BPADZp; BPZpGDBD | Hampel et al., 2015 | RRID:BDSC_79603 | spGAL4 control |
| Genetic reagent (D. melanogaster) | BDPLexA | Pfeiffer et al., 2010 | RRID:BDSC_77691 | |
| Genetic reagent (D. melanogaster) | 20XUAS-IVS-CsChrimson-mVenus | Klapoetke et al., 2014 | RRID:BDSC_55134 | |
| Genetic reagent (D. melanogaster) | MCFO-5 | Nern et al., 2015 | RRID:BDSC_64089 | |
| Genetic reagent (D. melanogaster) | MCFO-3 | Nern et al., 2015 | RRID:BDSC_64087 | |
| Genetic reagent (D. melanogaster) | 13XLexAop2-IVS-CsChrimson-mVenus | Klapoetke et al., 2014 | RRID:BDSC_55137 | |
| Antibody | Anti-GFP (Rabbit polyclonal) | Thermo Fisher Scientific | Cat# A-11122, RRID:AB_221569 | IF(1:500) |
| Antibody | Anti-Brp (Mouse monoclonal) | DSHB | Cat# nc82, RRID:AB_2314866 | IF(1:50) |
| Antibody | Anti-FLAG (Rat monoclonal) | Novus Biologicals | Cat# NBP1-06712, RRID:AB_1625981 | IF(1:300) |
| Antibody | Anti-HA (Rabbit monoclonal) | Cell Signaling Technology | Cat# 3724, RRID:AB_1549585 | IF(1:500) |
| Antibody | Anti-V5 (Mouse monoclonal) | Bio-Rad | Cat# MCA1360, RRID:AB_322378 | IF(1:300) |
| Antibody | Anti-Rabbit AF488 (Goat polyclonal) | Thermo Fisher Scientific | Cat# A-11034, RRID:AB_2576217 | IF(1:500) |
| Antibody | Anti-Mouse AF568 (Goat polyclonal) | Thermo Fisher Scientific | Cat# A-11031, RRID:AB_144696 | IF(1:500) |
| Antibody | Anti-Rat AF633 (Goat polyclonal) | Thermo Fisher Scientific | Cat# A-21094, RRID:AB_2535749 | IF(1:500) |
| Chemical compound, drug | Paraformaldehyde 20% | Electron Microscopy Sciences | Cat# 15713 | |
| Chemical compound, drug | DiD solid | Thermo Fisher Scientific | Cat# 07757 | |
| Chemical compound, drug | all-trans-Retinal | Toronto Research Chemicals | Cat# R240000 | |
| Software, algorithm | neuTube | Feng et al., 2015 | https://www.neutracing.com/ | |
| Software, algorithm | Vcode | Hagedorn et al., 2008 | http://social.cs.uiuc.edu/projects/vcode.html | |
| Software, algorithm | Fiji | Schindelin et al., 2012 | http://fiji.sc/ | |
| Software, algorithm | R | R Core Team | RRID:SCR_001905 | https://www.r-project.org/ |
| Software, algorithm | CMTK | Jefferis et al., 2007 | https://www.nitrc.org/projects/cmtk/ | |
| Software, algorithm | FluoRender | Wan et al., 2012 | http://www.sci.utah.edu/software/fluorender.html | |
| Software, algorithm | Blender version 2.79 | Blender Online Community | RRID:SCR_008606 | https://www.blender.org/download/releases/2-79/ |
| Software, algorithm | MATLAB | MathWorks Inc, Natick, MA, USA | RRID:SCR_001622 | |
| Software, algorithm | natverse | Bates et al., 2020 | http://natverse.org/ | |
| Software, algorithm | Cytoscape | Shannon et al., 2003 | https://cytoscape.org/ |
| Type | LM counts | EM left side | EM right side |
|---|---|---|---|
| Ant | 18-22 | 20 | 21 |
| Fr | 4-7 | 6 | 7 |
| FrOr | 4-6 | 6 | 7 |
| Or | 3 | 3 | 3 |
| Oc | 1 | 1 | 1 |
| InOc | 3-4 | 3 | 3 |
| Vib | 13-18 | 18 | 19 |
| InOm | 607-645 | 555 | 557 |
| vOcci/vPoOr | 21-33 | 26 | 26 |
| MaPa | 14-18 | 15 | 15 |
| Hau | 5 | 5 | 5 |
| Taste | 31-42 | 35 | 29 |
| Vt/PoOc | 4-5 | 5 | 5 |
| dPoOr | 5-9 | 4 | 8 |
| dOcci | 2-5 | 3 | 3 |
Additional files
-
Supplementary file 1
Quantification of bristle populations on male and female heads.
Table 1. Bristles counted using white light-illuminated male and female heads. Results of a two-tailed t-test comparing male and female bristle numbers are also shown. Table 2 shows counts of PoOr bristles innervated by BMNs that project through the OcciNv (dPoOr bristles) or the EyeNv (vPoOr bristles). BMNs were labeled by R52A06-GAL4. Table 3 shows vOcci bristles counted based on having an associated BMN labeled in the pBMN-spGAL4 (R28D07-AD ∩ VT050279-DBD) pattern (males). Table 4 shows a summary of published bristle numbers.
- https://cdn.elifesciences.org/articles/87602/elife-87602-supp1-v1.xlsx
-
Supplementary file 2
Table of download links for light microscopy image stacks used in this manuscript.
Image stacks are stored in the Brain Image Library (BIL), and were used to generate panels for Figure 1, Figure 2, Figure 3, Figure 4, and figure supplements for these figures. Rows in the spreadsheet correspond to each image stack. Columns provide information about each stack including: figure panels that each image stack contributed to, image stack title, DOI for each stack (link provides metadata for each stack and file download link), image stack file name, genotype of imaged fly, and information about image stack.
- https://cdn.elifesciences.org/articles/87602/elife-87602-supp2-v1.xlsx
-
Supplementary file 3
Table of electron microscopy (EM)-reconstructed bristle mechanosensory neurons (BMNs), Johnston’s organ neurons (JONs), and taste peg mechanosensory neurons (TPMNs).
Table includes FlyWire.ai neuron XYZ coordinates and IDs, nerve groups, names, types, NBLAST clusters, and cosine similarity clusters.
- https://cdn.elifesciences.org/articles/87602/elife-87602-supp3-v1.xlsx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/87602/elife-87602-mdarchecklist1-v1.pdf





