Protein Tracking: Everything, everywhere, almost at once
Proteins play a role in almost all cellular processes and are essential for maintaining life across species and organisms. This means that their aberrant function is a major cause of disease. If one could look directly inside cells, they would see a seemingly chaotic scene of proteins continuously moving around. The motion of each protein is heterogeneous in time and space, and linked to its role within the cell. It is also heavily influenced by the local cellular environment and interactions with other molecules (Lippincott-Schwartz et al., 2001; Kusumi et al., 2014).
Conventional research techniques average the behavior of a large number of unsynchronized molecules, and thus fail to account for these variable factors, which are essential for understanding the biology of proteins. This is where single-molecule tracking methods come into play (Chenouard et al., 2014 ). Traditional ways for tracking individual molecules rely on advanced fluorescence microscopy, single-particle tracking and super-resolution imaging to directly observe the movement and interactions of proteins (Kusumi et al., 2014; Sahl et al., 2017; Shen et al., 2017).
These approaches provide the required spatiotemporal resolution, but typically can only analyze a few cells under limited conditions, offering a narrow glimpse of the vast and dynamic world of proteins. Although high-throughput microscopy has become much refined, scaling single-particle tracking remains a challenge (Park et al., 2023; Malle et al., 2022). A method that could record the performance of every single protein inside millions of individual cells, as well as thousands of molecular compounds, and analyze how they move, interact and respond to therapeutics, would be a major scientific breakthrough – one that may soon be a reality.
Now, in eLife, Hilary Beck and colleagues at Eikon Therapeutics and University of California Berkeley – including David McSwiggen as first author – report a high-throughput tracking (htSMT) platform that makes it possible to observe and analyze the behavior and movement of single proteins and molecules on an unprecedented scale (McSwiggen et al., 2023). The platform involves a robotic system capable of autonomously handling reagents and collecting sequential microscopy movies that are then computationally processed to obtain the trajectories of individual proteins within cells and even cellular compartments. This system allows users to image over a million cells, track thousands of individual proteins per cell, and screen thousands of compounds in a single day.
McSwiggen et al. then tested the platform on estrogen receptors and investigated how over 5,000 compounds affected their motion, analyzing hundreds of thousands of cells twice in a single day (Figure 1). This revealed a new correlation between the dynamics of estrogen receptors and the ability of their antagonists to suppress the growth of cancer cells, which conventual methods have failed to detect previously. Moreover, the htSMT platform also revealed whether the tested molecules affect estrogen receptors directly or indirectly through other biological targets that are known to modify the receptor. This provides an unprecedented and unbiased analysis of a complex biological pathway.
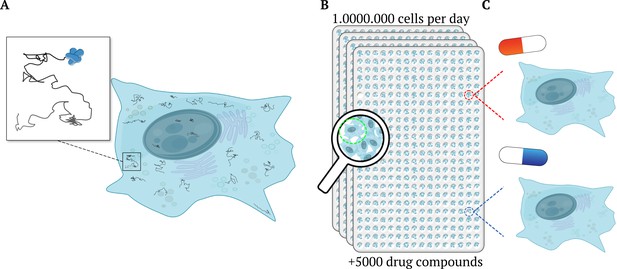
High-throughput single-molecule tracking of proteins across millions of cells.
(A) Schematic illustration of a cell (blue shape) as taken from a high-throughput single-molecule tracking (htSMT) assay (inset), which tracks the motion of multiple proteins within individual cells (squiggly lines). (B) The platform can record the movement of thousands of individual, heterogeneous proteins per cell in over a million cells per day. It does this by automatically collecting a series of images from 384-well plates mounted on a microscope. Each well contains multiples cells and constitutes an independent experiment, enabling researchers to investigate different cell types, and test the effects of various drugs and other molecules and/ or proteins. (C) The htSMT results from cells treated with different drugs (indicated as a red or blue pill) can then be used to assess which treatment is likely to work best.
Image credit: Jacob Kæstel-Hansen and Nikos S. Hatzakis. The figure was – with large modifications – generated with elements from Servier Medical Art and Scidraw.io (10.5281/zenodo.3926549; CC BY 4.0).
Overall, the htSMT platform paves the way for a new era in cellular biology and pharmacology, enabling large-scale, automated observations of how proteins move and interact across millions of cells within 24 hours – a feat that until recently remained in the realm of fantasy. This profound increase in scale, together with advanced analytic tools (Muñoz-Gil et al., 2021; Pinholt et al., 2021), promises to unlock even more unresolved information about complex biological pathways, such as those associated with the estrogen receptor. Adapting htSMT to other proteins and cell systems could help construct unique libraries that ultimately link movement to function. Exploiting the full potential of htSMT will further our understanding of the intricate processes occurring within cells and how protein motion contributes to – and depends on – cellular function. Ultimately this could help researchers design new pharmaceutical treatments for controlling certain diseases.
References
-
Objective comparison of particle tracking methodsNature Methods 11:281–289.https://doi.org/10.1038/nmeth.2808
-
Tracking single molecules at work in living cellsNature Chemical Biology 10:524–532.https://doi.org/10.1038/nchembio.1558
-
Studying protein dynamics in living cellsNature Reviews. Molecular Cell Biology 2:444–456.https://doi.org/10.1038/35073068
-
Objective comparison of methods to decode anomalous diffusionNature Communications 12:6253.https://doi.org/10.1038/s41467-021-26320-w
-
Fluorescence nanoscopy in cell biologyNature Reviews Molecular Cell Biology 18:685–701.https://doi.org/10.1038/nrm.2017.71
-
Single particle tracking: from theory to biophysical applicationsChemical Reviews 117:7331–7376.https://doi.org/10.1021/acs.chemrev.6b00815
Article and author information
Author details
Publication history
- Version of Record published: January 29, 2024 (version 1)
Copyright
© 2024, Kæstel-Hansen and Hatzakis
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 2,149
- views
-
- 148
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Cell Biology
Erythropoiesis and megakaryopoiesis are stringently regulated by signaling pathways. However, the precise molecular mechanisms through which signaling pathways regulate key transcription factors controlling erythropoiesis and megakaryopoiesis remain partially understood. Herein, we identified heat shock cognate B (HSCB), which is well known for its iron–sulfur cluster delivery function, as an indispensable protein for friend of GATA 1 (FOG1) nuclear translocation during erythropoiesis of K562 human erythroleukemia cells and cord-blood-derived human CD34+CD90+hematopoietic stem cells (HSCs), as well as during megakaryopoiesis of the CD34+CD90+HSCs. Mechanistically, HSCB could be phosphorylated by phosphoinositol-3-kinase (PI3K) to bind with and mediate the proteasomal degradation of transforming acidic coiled-coil containing protein 3 (TACC3), which otherwise detained FOG1 in the cytoplasm, thereby facilitating FOG1 nuclear translocation. Given that PI3K is activated during both erythropoiesis and megakaryopoiesis, and that FOG1 is a key transcription factor for these processes, our findings elucidate an important, previously unrecognized iron–sulfur cluster delivery independent function of HSCB in erythropoiesis and megakaryopoiesis.
-
- Biochemistry and Chemical Biology
- Cell Biology
Hibernation is a period of metabolic suppression utilized by many small and large mammal species to survive during winter periods. As the underlying cellular and molecular mechanisms remain incompletely understood, our study aimed to determine whether skeletal muscle myosin and its metabolic efficiency undergo alterations during hibernation to optimize energy utilization. We isolated muscle fibers from small hibernators, Ictidomys tridecemlineatus and Eliomys quercinus and larger hibernators, Ursus arctos and Ursus americanus. We then conducted loaded Mant-ATP chase experiments alongside X-ray diffraction to measure resting myosin dynamics and its ATP demand. In parallel, we performed multiple proteomics analyses. Our results showed a preservation of myosin structure in U. arctos and U. americanus during hibernation, whilst in I. tridecemlineatus and E. quercinus, changes in myosin metabolic states during torpor unexpectedly led to higher levels in energy expenditure of type II, fast-twitch muscle fibers at ambient lab temperatures (20 °C). Upon repeating loaded Mant-ATP chase experiments at 8 °C (near the body temperature of torpid animals), we found that myosin ATP consumption in type II muscle fibers was reduced by 77–107% during torpor compared to active periods. Additionally, we observed Myh2 hyper-phosphorylation during torpor in I. tridecemilineatus, which was predicted to stabilize the myosin molecule. This may act as a potential molecular mechanism mitigating myosin-associated increases in skeletal muscle energy expenditure during periods of torpor in response to cold exposure. Altogether, we demonstrate that resting myosin is altered in hibernating mammals, contributing to significant changes to the ATP consumption of skeletal muscle. Additionally, we observe that it is further altered in response to cold exposure and highlight myosin as a potentially contributor to skeletal muscle non-shivering thermogenesis.






